| Enzyme / Gene |
Activity |
Object |
References |
|---|
Enzyme name: RfaF
UniProt ID: P37692 
CAZy family: GT9
Gene name: waaF / rfaF
Gene GenBank ID: 948135*  | Synthesized dimer: aLDmanHepp(1-3)aLDmanHepp
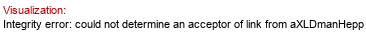aXLDmanHepp)
Donor (ID 34402): bXLDmanHepp(1-P-P-5)xXnucA
Acceptor (ID 34411): l?3HOMyr(1-2)[lXLau(1-3)l?3HOMyr(1-2)[aXKdo(2-4)[aXLDmanHepp(1-5)]aXKdo(2-6),P-4),lXMyr(1-3)l?3HOMyr(1-3)]bDGlcpN(1-6),l?3HOMyr(1-3)]aDGlcpN(1-P
Status: evidence in vitro
Confirmation methods: in vitro (crude extract), mutation (dual, rfaC + rfaF)
ID: 2101 Notes: WaaA, WaaC, WaaF, and WaaQ participate in the synthesis of Ac(1-2)b?GlcpN(1-7)?XLDmanHepp(1-6)??Glcp(1-2)??Glcp(1-3)[??Gal?(1-6)]??Glcp(1-3)[aXLDmanHepp(1-7)]aXLDmanHepp(1-3)aXLDmanHepp(1-5)[<>[%xXEtN(1-7)]aXKdo(2-4)]aXKdo(2-6)[lXLau(1-3)l?3HOMyr(1-2),P-4),lXMyr(1-3)l?3HOMyr(1-3)]bDGlcpN(1-6)[l?3HOMyr(1-3),l?3HOMyr(1-2)]aDGlcpN(1-P.
| Organism (ID 23): Escherichia coli K12
Full structure (ID 34412):
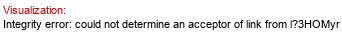[lXLau(1-3)l?3HOMyr(1-2)[aXKdo(2-4)[aXLDmanHepp(1-3)aXLDmanHepp(1-5)]aXKdo(2-6),P-4),lXMyr(1-3)l?3HOMyr(1-3)]bDGlcpN(1-6),l?3HOMyr(1-3)]aDGlcpN(1-P&highlight=5~3~aXLDmanHep)
Molecule role (analog of): core | Gronow et al. 2000
DOI: 10.1046/j.1432-1327.2000.01754.x
Brabetz et al. 1997
DOI: 10.1111/j.1432-1033.1997.00716.x
Gronow et al. 2001
DOI: 10.1177/09680519010070040701
|
Synthesized dimer: aLDmanHepp(1-3)aLDmanHepp
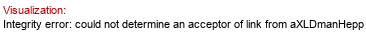aXLDmanHepp)
Donor (ID 34402): bXLDmanHepp(1-P-P-5)xXnucA
Acceptor (ID 34413): l?3HOMyr(1-2)[l?3HOMyr(1-2)[aXKdo(2-4)[aXLDmanHepp(1-5)]aXKdo(2-6),P-4),l?3HOMyr(1-3)]bDGlcpN(1-6),l?3HOMyr(1-3)]aDGlcpN(1-P
Status: evidence in vitro
Confirmation methods: in vitro (crude extract)
ID: 2102 Notes: WaaA, WaaC, WaaF, and WaaQ participate in the synthesis of Ac(1-2)b?GlcpN(1-7)?XLDmanHepp(1-6)??Glcp(1-2)??Glcp(1-3)[??Gal?(1-6)]??Glcp(1-3)[aXLDmanHepp(1-7)]aXLDmanHepp(1-3)aXLDmanHepp(1-5)[<>[%xXEtN(1-7)]aXKdo(2-4)]aXKdo(2-6)[lXLau(1-3)l?3HOMyr(1-2),P-4),lXMyr(1-3)l?3HOMyr(1-3)]bDGlcpN(1-6)[l?3HOMyr(1-3),l?3HOMyr(1-2)]aDGlcpN(1-P.
| Organism (ID 23): Escherichia coli K12
Full structure (ID 34414):
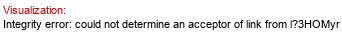[l?3HOMyr(1-2)[aXKdo(2-4)[aXLDmanHepp(1-3)aXLDmanHepp(1-5)]aXKdo(2-6),P-4),l?3HOMyr(1-3)]bDGlcpN(1-6),l?3HOMyr(1-3)]aDGlcpN(1-P&highlight=5~3~aXLDmanHep)
Molecule role (analog of): core | Gronow et al. 2000
DOI: 10.1046/j.1432-1327.2000.01754.x
Gronow et al. 2001
DOI: 10.1177/09680519010070040701
|
#GTR ID Enzyme name Enzyme group Enzyme Genbank ID Derived ID mark Enzyme Uniprot ID Derived ID mark CAZY family Gene name Gene Genbank ID Derived ID mark Gene cluster Full structure CSDB structure ID Side product CSDB structure ID Full structure Full structure is fragment (0|1) Synthesized bond Synthesized bond location (from root ~ subst position) Donor structure ID Substrate structure Confirmation status Methods used Notes Molecule role Molecule is a model of role (0|1) Molecule is a precursor of role (0|1) Main CSDB record ID Additional CSDB record IDs CSDB organism IDs Organism names Organs and tissues References (;-separated)
2101 RfaF P37692 GT9 waaF / rfaF 948135 * 34412 l?3HOMyr(1-2)[lXLau(1-3)l?3HOMyr(1-2)[aXKdo(2-4)[aXLDmanHepp(1-3)aXLDmanHepp(1-5)]aXKdo(2-6),P-4),lXMyr(1-3)l?3HOMyr(1-3)]bDGlcpN(1-6),l?3HOMyr(1-3)]aDGlcpN(1-P 0 aXLDmanHepp(1-3)aXLDmanHepp 5~3 bXLDmanHepp(1-P-P-5)xXnucA l?3HOMyr(1-2)[lXLau(1-3)l?3HOMyr(1-2)[aXKdo(2-4)[aXLDmanHepp(1-5)]aXKdo(2-6),P-4),lXMyr(1-3)l?3HOMyr(1-3)]bDGlcpN(1-6),l?3HOMyr(1-3)]aDGlcpN(1-P in vitro in vitro (crude extract), mutation (dual, rfaC + rfaF) WaaA, WaaC, WaaF, and WaaQ participate in the synthesis of Ac(1-2)b?GlcpN(1-7)?XLDmanHepp(1-6)??Glcp(1-2)??Glcp(1-3)[??Gal?(1-6)]??Glcp(1-3)[aXLDmanHepp(1-7)]aXLDmanHepp(1-3)aXLDmanHepp(1-5)[<>[%xXEtN(1-7)]aXKdo(2-4)]aXKdo(2-6)[lXLau(1-3)l?3HOMyr(1-2),P-4),lXMyr(1-3)l?3HOMyr(1-3)]bDGlcpN(1-6)[l?3HOMyr(1-3),l?3HOMyr(1-2)]aDGlcpN(1-P. core 1 0 23 Escherichia coli K12 Gronow et al. 2000 (doi: 10.1046/j.1432-1327.2000.01754.x); Brabetz et al. 1997 (doi: 10.1111/j.1432-1033.1997.00716.x); Gronow et al. 2001 (doi: 10.1177/09680519010070040701)
2102 RfaF P37692 GT9 waaF / rfaF 948135 * 34414 l?3HOMyr(1-2)[l?3HOMyr(1-2)[aXKdo(2-4)[aXLDmanHepp(1-3)aXLDmanHepp(1-5)]aXKdo(2-6),P-4),l?3HOMyr(1-3)]bDGlcpN(1-6),l?3HOMyr(1-3)]aDGlcpN(1-P 0 aXLDmanHepp(1-3)aXLDmanHepp 5~3 bXLDmanHepp(1-P-P-5)xXnucA l?3HOMyr(1-2)[l?3HOMyr(1-2)[aXKdo(2-4)[aXLDmanHepp(1-5)]aXKdo(2-6),P-4),l?3HOMyr(1-3)]bDGlcpN(1-6),l?3HOMyr(1-3)]aDGlcpN(1-P in vitro in vitro (crude extract) WaaA, WaaC, WaaF, and WaaQ participate in the synthesis of Ac(1-2)b?GlcpN(1-7)?XLDmanHepp(1-6)??Glcp(1-2)??Glcp(1-3)[??Gal?(1-6)]??Glcp(1-3)[aXLDmanHepp(1-7)]aXLDmanHepp(1-3)aXLDmanHepp(1-5)[<>[%xXEtN(1-7)]aXKdo(2-4)]aXKdo(2-6)[lXLau(1-3)l?3HOMyr(1-2),P-4),lXMyr(1-3)l?3HOMyr(1-3)]bDGlcpN(1-6)[l?3HOMyr(1-3),l?3HOMyr(1-2)]aDGlcpN(1-P. core 1 0 23 Escherichia coli K12 Gronow et al. 2000 (doi: 10.1046/j.1432-1327.2000.01754.x); Gronow et al. 2001 (doi: 10.1177/09680519010070040701)

