 Found 4 records.
Displayed records from 1 to 4
Found 4 records.
Displayed records from 1 to 4
Expand all records
Collapse all records
Show all as text (SweetDB notation)
Show all graphically (SNFG notation)
Torrenegra R, Robles J, Pedrozo J, Pescador B
A new diglycoside of diterpene from Ageratina vacciniaefolia
Molecules 4 (1999)
ID M94
|
b-D-Glcp-(1-19)-+
|
b-D-Glcp-(1-17)-Subst
Subst = 16β,17-dihydroxy-ent-kaurane-19-oic acid = SMILES O{17}C{16}[C@@]1(O)[C@@H]2C[C@]3(CC[C@]4([C@](C)(CCC[C@@]4([C@]3(CC2)[H])C){19}C(O)=O)[H])C1 |
Show graphically |
Ageratina vacciniaefolia

(Ancestor NCBI TaxID 102749,
species name lookup)
Taxonomic group: plant / Streptophyta
(Phylum: Streptophyta)
Organ / tissue: leaf,
flower
The structure was elucidated in this paperPublication DOI: 10.3390/m94Journal NLM ID: 100964009Publisher: Basel, Switzerland: MDPI
Correspondence: Torrenegra R <rtorrene

javercol.javeriana.edu.co>
Institutions: Pontificia Universidad Javeriana, Grupo de Investigación Fitoquímica GIFUJ, Santafé de Bogotá, Colombia
glycoside, diterpene, Ageratina vacciniaefolia
Structure type: oligomer ; 499
Location inside paper: abstract, table 1
Compound class: diterpenoid glycoside
Contained glycoepitopes: IEDB_142488,IEDB_146664,IEDB_983931,SB_192
Methods: 13C NMR, 1H NMR, enzymatic hydrolysis, optical rotation measurement, CID-MS, HMBC, COSY, NOESY
Comments, role: NMR temperature was not specified
NCBI Taxonomy refs (TaxIDs): 102749
Show glycosyltransferases
NMR conditions: in CD3OD
[as TSV]
13C NMR data:
Linkage Residue C1 C2 C3 C4 C5 C6 C7 C8 C9 C10 C11 C12 C13 C14 C15 C16 C17 C18 C19 C20
17 bDGlcp 95.6 ? ? ? ? ?
19 bDGlcp 98.5 ? ? ? ? ?
Subst 41.8 19.7 39.1 45.1 57.3 23.2 43.1 46.0 58.5 40.9 20.1 26.8 44.2 37.6 51.4 91.4 63.6 29.1 178.2 16.4
1H NMR data: present in publication
|
13C NMR data:
| Linkage | Residue | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | C11 | C12 | C13 | C14 | C15 | C16 | C17 | C18 | C19 | C20 |
|---|
| 17 | bDGlcp | 95.6 | ? | ? | ? | ? | ? | |
| 19 | bDGlcp | 98.5 | ? | ? | ? | ? | ? | |
| | Subst | 41.8 | 19.7 | 39.1 | 45.1 | 57.3 | 23.2 | 43.1 | 46.0 | 58.5 | 40.9 | 20.1 | 26.8 | 44.2 | 37.6 | 51.4 | 91.4 | 63.6 | 29.1 | 178.2 | 16.4 |
|
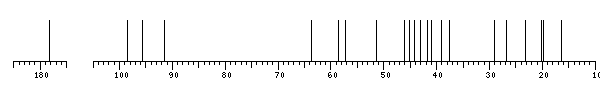
The spectrum also has 10 signals at unknown positions (not plotted). |
There is only one chemically distinct structure:
Expand this record
Collapse this record
Cifuente DA, Tonn CE, Giordano OS
Two new labdane diterpene glycoside from flowers of Bacchris medulosa DC
Natural Product Letters 5(3) (2000)
386-387
|
Ang-(1-3)-+
|
b-L-Rhap-(1-2)-Subst15Ac
Subst = labdane-type diterpene 1 = SMILES C[C@]12[C@H](CC=C(C)[C@H]2CC/C(C)=C/{15}CO)C(C)(C){3}[C@H](O){2}[C@@H](O)C1 |
Show graphically |
Baccharis medullosa

(Ancestor NCBI TaxID 41487,
species name lookup)
Taxonomic group: plant / Streptophyta
(Phylum: Streptophyta)
Organ / tissue: flower
The structure was elucidated in this paperPublication DOI: 10.3390/50300386Journal NLM ID: 9315615Publisher: Taylor & Francis Health Sciences
Correspondence: cifuente

unsl.edu.ar
Institutions: Intequi-Conicet-Facultad de Química, Bioquímica y Farmacia, Universidad Nacional de San Luis, Chacabuco y Pedernera, Argentina
Two new labdane-type diterpene glycoside, were isolated from the flowers of Baccharis medullosa DC (Asteraceae). Structures of these compounds were established by application of various spectroscopic techniques.
Diterpene glycosides, Bacchris medulosa
Structure type: monomer
Location inside paper: p. 386, structure 1
Compound class: glycoside, diterpenoid glycoside
Contained glycoepitopes: IEDB_225177,IEDB_885823
Methods: 13C NMR, 1H NMR, NMR-2D, acid hydrolysis, GC, extraction, CC, derivatization
Related record ID(s): 65164
NCBI Taxonomy refs (TaxIDs): 41487
Show glycosyltransferases
There is only one chemically distinct structure:
Expand this record
Collapse this record
Cifuente DA, Tonn CE, Giordano OS
Two new labdane diterpene glycoside from flowers of Bacchris medulosa DC
Natural Product Letters 5(3) (2000)
386-387
|
Ang-(1-3)-+
|
b-L-Rhap-(1-2)-Subst
Subst = labdane-type diterpene 1 = SMILES C[C@]12[C@H](CC=C(C)[C@H]2CC/C(C)=C/{15}CO)C(C)(C){3}[C@H](O){2}[C@@H](O)C1 |
Show graphically |
Baccharis medullosa

(Ancestor NCBI TaxID 41487,
species name lookup)
Taxonomic group: plant / Streptophyta
(Phylum: Streptophyta)
Organ / tissue: flower
The structure was elucidated in this paperPublication DOI: 10.3390/50300386Journal NLM ID: 9315615Publisher: Taylor & Francis Health Sciences
Correspondence: cifuente

unsl.edu.ar
Institutions: Intequi-Conicet-Facultad de Química, Bioquímica y Farmacia, Universidad Nacional de San Luis, Chacabuco y Pedernera, Argentina
Two new labdane-type diterpene glycoside, were isolated from the flowers of Baccharis medullosa DC (Asteraceae). Structures of these compounds were established by application of various spectroscopic techniques.
Diterpene glycosides, Bacchris medulosa
Structure type: monomer
Location inside paper: p. 386, structure 2
Compound class: glycoside, diterpenoid glycoside
Contained glycoepitopes: IEDB_225177,IEDB_885823
Methods: 13C NMR, 1H NMR, NMR-2D, acid hydrolysis, GC, extraction, CC, derivatization
Related record ID(s): 65163
NCBI Taxonomy refs (TaxIDs): 41487
Show glycosyltransferases
There is only one chemically distinct structure:
Expand this record
Collapse this record
Liu H-M, Yan X, Kiuchi F, Liu Z
A new diterpene glycoside from Rabdosia rubescens
Chemical and Pharmaceutical Bulletin 48(1) (2000)
148-149
|
b-D-Glcp-(1-1)-Subst
Subst = enmenol = SMILES CC1(C)CC{1}[C@H](O)[C@]23[C@@H]1{6}[C@H](O){7}[C@](OC3)(O)[C@]45[C@H]2CC[C@@H](C([C@H]{15}5O)=C){14}[C@H]4O |
Show graphically |
Rabdosia rubescens
 (later renamed to: Isodon rubescens)
(later renamed to: Isodon rubescens)
(NCBI TaxID 587669,
species name lookup)
Taxonomic group: plant / Streptophyta
(Phylum: Streptophyta)
Organ / tissue: leaf
The structure was elucidated in this paperNCBI PubMed ID: 10705493Publication DOI: 10.1248/cpb.48.148Journal NLM ID: 0377775Publisher: Pharmaceutical Society Of Japan
Institutions: Department of Chemistry, Zhengzhou University, Zhengzhou, China, Faculty of Pharmaceutical Sciences, Kanazawa University, Kanazawa, Japan
A new ent-kaurene β-D-glucoside was isolated from Rabdosia rubescens, together with the known compounds oridonin, ponicidin, and pedalitin. The structure of new compound was determined, on the basis of spectral date and X-ray crystallographic analysis, to be ent-7β, 20-epoxy-kaur-16-ene-1β, 6α, 7α, 14α, 15α-pentanol 1-O-β-D-glucopyranoside.
crystal structure, diterpene, Rabdosia rubescens, ent-kaurene, diterpene glycoside
Structure type: monomer
Location inside paper: p. 148, structure 1
Compound class: glycoside, diterpenoid glycoside
Contained glycoepitopes: IEDB_142488,IEDB_146664,IEDB_983931,SB_192
Methods: 13C NMR, 1H NMR, TLC, ESI-MS, HPLC, extraction, derivatization, evaporation, HR-EI-MS
NCBI Taxonomy refs (TaxIDs): 587669
Show glycosyltransferases
There is only one chemically distinct structure:
Expand this record
Collapse this record
Total list of record IDs on all result pages of the current query:
Execution: <1 sec
 report error
report error report error
report error
 report error
report error
 report error
report error
 report error
report error (later renamed to: Isodon rubescens)
(later renamed to: Isodon rubescens)